Tag: originator biologic
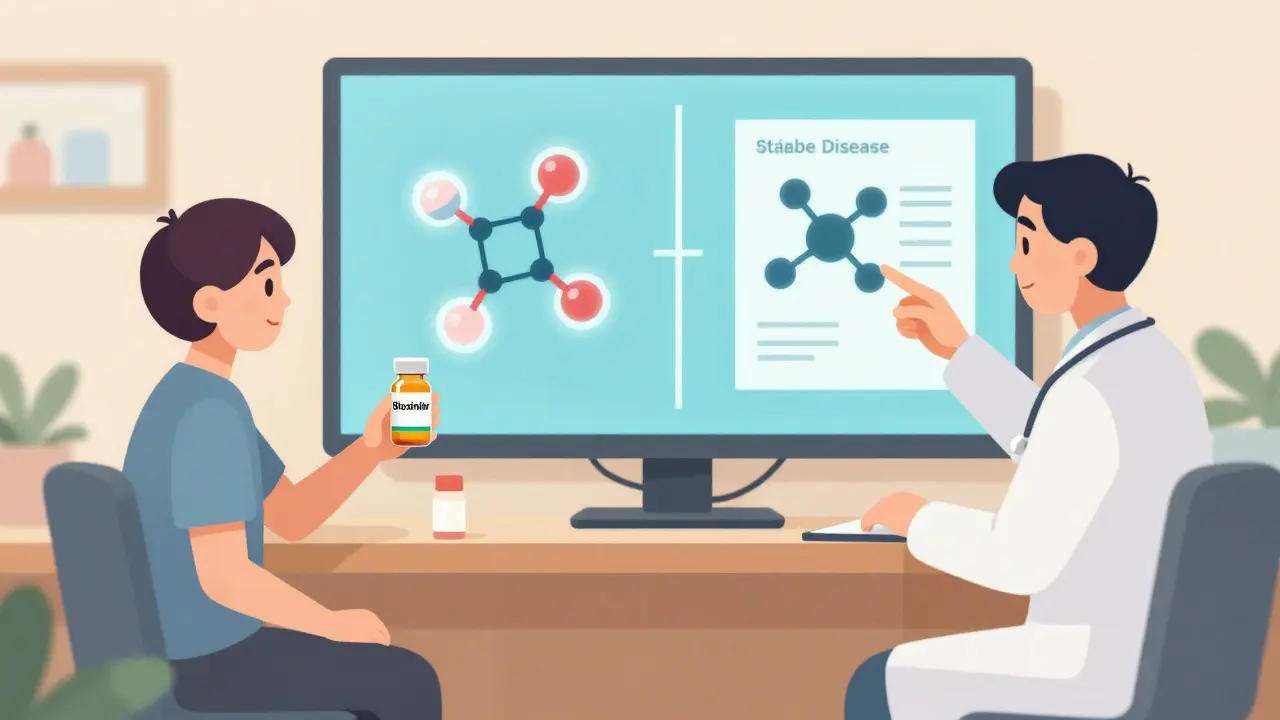
Biosimilar Switching: What Happens When You Change from Originator Biologic
Switching from an originator biologic to a biosimilar is safe for most patients with stable autoimmune conditions. Learn what really happens during the switch, why some people stop treatment, and how to make the transition work for you.
view morerecent posts

Learn how to verify online pharmacy licenses to avoid counterfeit drugs. Step-by-step guide to using state and NABP verification systems for safe medication purchases.
Published ON: 15 Feb
Polycystic kidney disease is a genetic disorder causing cysts to grow in the kidneys, leading to kidney failure. Learn how ADPKD and ARPKD work, how to manage symptoms, and what new treatments are on the horizon.
Published ON: 7 Feb
Learn how post-marketing studies track drug safety after approval, using FAERS, Sentinel, and real-world data to catch hidden risks. Understand the systems, challenges, and future of pharmacovigilance.
Published ON: 11 Feb
Hyponatremia and hypernatremia are common, dangerous complications in kidney disease. Learn how sodium imbalances happen, why standard treatments fail, and what actually works to protect your health.
Published ON: 8 Feb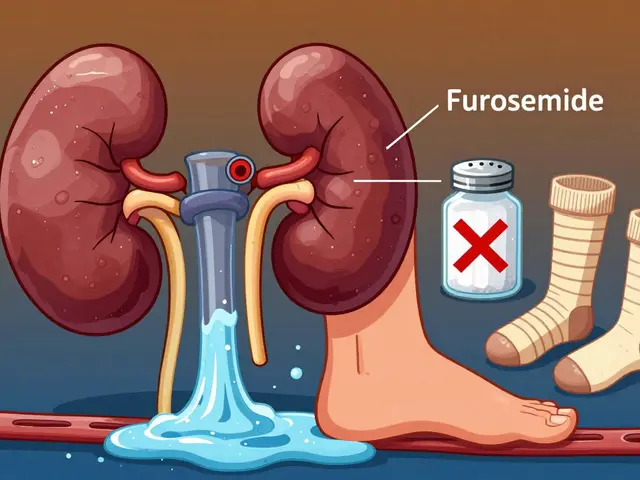
Edema in CKD is caused by fluid buildup from failing kidneys. Managing it requires a three-part strategy: the right diuretics, strict salt restriction, and compression therapy. Learn how each works-and how to use them safely.
Published ON: 7 Feb