When you’ve been on a biologic drug for years - say, adalimumab or infliximab - to manage rheumatoid arthritis, Crohn’s disease, or psoriasis, switching to a biosimilar isn’t just a paperwork change. It’s a real shift in your treatment, and it comes with real questions. Biosimilar switching is now common in clinics across the U.S. and Europe, but what actually happens when you go from the original brand-name drug to a biosimilar? Do you lose control of your disease? Do side effects pop up out of nowhere? Is it safe to switch more than once?
What Exactly Is a Biosimilar?
A biosimilar isn’t a generic. Generics are exact copies of small-molecule drugs like aspirin or metformin. Biosimilars are copies of complex biologic drugs - proteins made from living cells. These aren’t simple chemicals. They’re large, intricate molecules that can vary slightly even between batches of the same originator drug. That’s why regulators don’t call them "identical." They call them "highly similar."
The U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA) require biosimilars to match the originator in structure, function, safety, and effectiveness. They must show no clinically meaningful differences. That means: if your disease is under control on Humira, switching to an approved biosimilar shouldn’t make your symptoms worse.
The first U.S. biosimilar, Zarxio (filgrastim-sndz), was approved in 2015. Since then, 37 biosimilars have cleared FDA review, mostly targeting tumor necrosis factor (TNF) inhibitors like infliximab and adalimumab - drugs that dominate biologic use in autoimmune diseases. By 2022, these TNF biosimilars made up about 70% of all biosimilar prescriptions in the U.S., according to IQVIA data.
What Happens When You Switch?
Real-world data from large studies shows that switching from an originator biologic to a biosimilar usually works just as well. The NOR-Switch study, which tracked 481 patients with inflammatory arthritis or IBD, found that after one year, 52.6% of those switched to the infliximab biosimilar CT-P13 stayed on treatment. That’s almost the same as the 60% who stayed on the original infliximab. The difference wasn’t statistically significant.
In inflammatory bowel disease (IBD), another study found that 90.6% of patients maintained remission after switching from CT-P13 to another biosimilar, SB2. Their fecal calprotectin levels - a marker of gut inflammation - stayed nearly identical before and after the switch.
For psoriasis, a Danish study tracked over 1,000 patients. One year after switching from originator adalimumab to a biosimilar, drug retention was 79%. For those who never switched, it was 81.3%. The tiny difference didn’t mean the biosimilar was worse - it just showed that some people stop treatment for reasons unrelated to effectiveness.
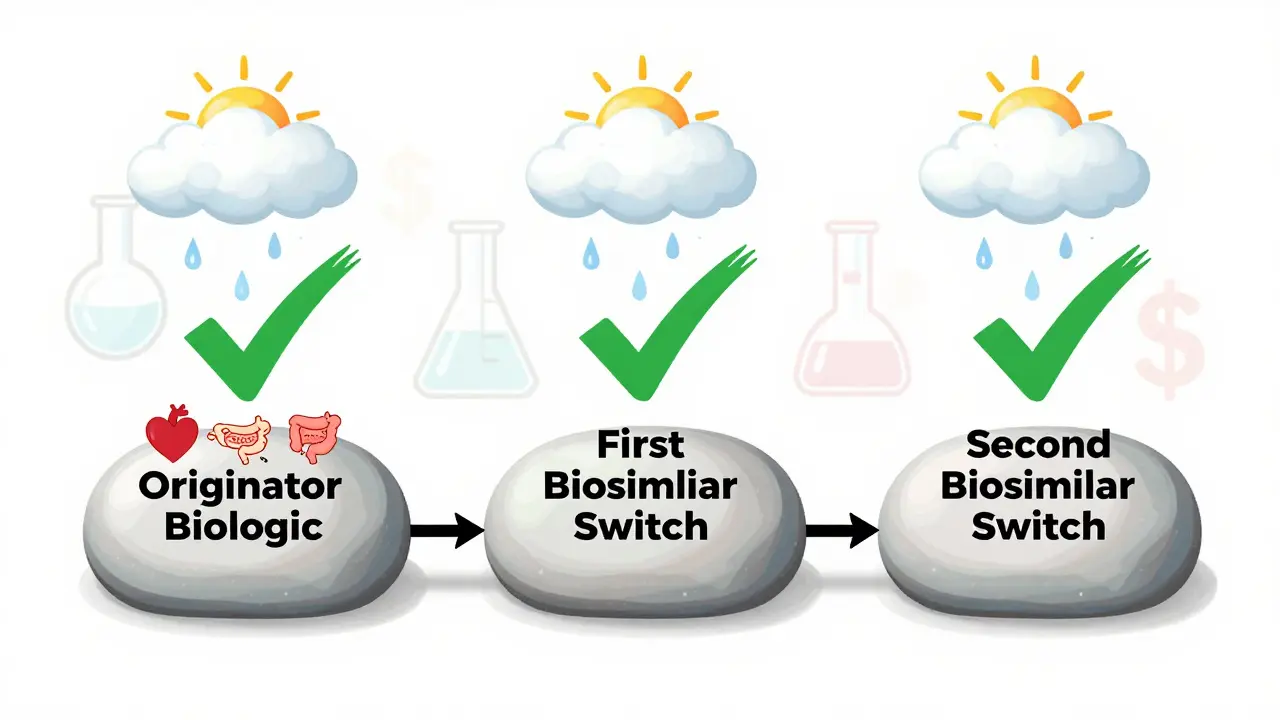
Even more reassuring: switching between biosimilars - say, from CT-P13 to SB2 - doesn’t appear to increase risk. A 2022 study of 140 patients who switched twice - first from originator to CT-P13, then to SB2 - found no rise in anti-drug antibodies, no drop in drug levels in the blood, and no spike in side effects. Trough levels (the lowest concentration of drug in your system before the next dose) stayed steady: 4.3 μg/mL before the first switch, 4.1 μg/mL after the second.
Why Do Some People Stop Taking Biosimilars?
It’s not always because the drug stopped working. A big reason is the nocebo effect - when people expect something bad to happen, their brain makes them feel it, even if the drug is doing exactly what it should.
A 2021 study in Frontiers in Psychology found that 32.7% of patients reported new or worsening symptoms after being switched to a biosimilar - even though lab tests and disease scores showed no actual change. Many of these patients believed the biosimilar was "inferior," even though they were told it was just as safe and effective.
Reddit communities are full of posts like: "I felt different after the switch," or "My joints hurt more since they changed my meds." But when doctors check their bloodwork, DAS28 scores, or PASI scores, everything looks normal. The symptoms are real to the patient - but they’re not caused by the drug failing.
Other reasons people stop include:
- Injection site reactions (7.8% in some adalimumab biosimilar studies)
- Perceived loss of effectiveness (12.6% in one etanercept switch study)
- Confusion or fear about the new drug
- Insurance forcing the switch without patient input
Actual immune reactions - like developing anti-drug antibodies that neutralize the drug - are rare. Studies show only about 1.7 events per 100 patient-years. That’s less than 2% of patients over a year. Most discontinuations aren’t due to biological failure. They’re due to perception.
Cost and Access: The Big Driver Behind Switching
Health systems are pushing biosimilars because they save money. Originator biologics like Humira cost over $2,000 per dose. Biosimilars launch at 15% to 35% less. In 2023, Humira biosimilars entered the U.S. market at a 35% discount, per CMS data. That’s billions in savings for insurers and patients.
By 2023, 85% of U.S. health plans had mandatory switch policies for certain biologics. That means if you’re on Humira and your plan switches you to a biosimilar, you don’t get a choice - unless your doctor files a medical exception.
Europe leads in adoption. In countries like Germany and Sweden, over 60% of patients on filgrastim get a biosimilar. The U.S. lags behind, with only 24% of infliximab prescriptions being biosimilars. Why? Patent lawsuits, rebate deals between drugmakers and insurers, and slower provider education.
When Is Switching Riskier?
Switching works best when your disease is stable. If your RA is under control (DAS28 score below 3.2), or your Crohn’s is in remission, switching is low-risk. But if you’re having a flare, your immune system is already on high alert. That’s not the time to change drugs.
Some experts warn against multiple switches. While one switch is well-supported, switching three or four times - say, originator → biosimilar A → biosimilar B → biosimilar C - hasn’t been studied long-term. A 2022 Spanish study found a 15.3% discontinuation rate after switching between two biosimilars in IBD patients, compared to 8.7% in those who didn’t switch. The authors didn’t find a drop in drug levels, but they did see more people quitting treatment.
Also, if you’ve had a serious reaction to the originator - like anaphylaxis or severe infusion reaction - switching may need extra caution. Your doctor might stick with the original drug.
How to Make the Switch Work for You
Switching doesn’t have to be scary. Here’s how to do it right:
- Ask for a conversation. Don’t just accept the switch. Talk to your rheumatologist or gastroenterologist. Ask: "Is this right for me?" and "What if I feel different?"
- Get educated. The PERFUSE study showed that just 20 minutes of pre-switch counseling cut discontinuation rates from 18% to 6.4%. Learn what biosimilars are, why they’re cheaper, and what to expect.
- Track your symptoms. Keep a simple journal: pain levels, fatigue, bowel movements, skin flare-ups. Don’t assume every new ache means the drug failed.
- Schedule a follow-up. Most experts recommend checking in at 3 months. Blood tests for drug levels and antibodies, plus disease activity scores, can tell you if the switch is working - or if something else is going on.
- Know your rights. If your insurance forces the switch and you’re unstable, your doctor can file an exception. You’re not powerless.

The Bottom Line
Switching from an originator biologic to a biosimilar is safe for most people. Decades of clinical data, from randomized trials to real-world registries, confirm that efficacy and safety are preserved. The FDA and EMA both agree: no meaningful difference exists between the two.
But safety isn’t just about lab results. It’s about trust. If you’re scared, you might feel worse - even if the drug is perfect. That’s why communication matters more than ever.
Biosimilars are here to stay. They’re making life-changing treatments affordable. But they’re not magic. They’re medicine. And like all medicine, they work best when you’re informed, involved, and listened to.
What About Interchangeability?
In 2024, the FDA approved the first interchangeable adalimumab biosimilar: Cyltezo. That means pharmacists can swap it for Humira without asking your doctor - just like generics.
But "interchangeable" doesn’t mean "better." It just means regulators have extra proof it works the same when switched back and forth. Only a few biosimilars have this designation so far. Most switches still require a prescriber’s order.
Don’t assume interchangeability means automatic safety. If you’ve had bad experiences with switches before, tell your pharmacist. You still have the right to refuse the substitution.
Is switching to a biosimilar safe for my condition?
Yes, for most people with stable autoimmune diseases like rheumatoid arthritis, psoriatic arthritis, Crohn’s disease, or plaque psoriasis. Large studies, including the NOR-Switch trial and multiple observational cohorts, show no increase in disease flares, serious side effects, or hospitalizations after switching from originator biologics to approved biosimilars. Safety is supported by the FDA and EMA based on over 120 clinical studies.
Will I have more side effects after switching?
Not necessarily. Most side effects - like injection site reactions or fatigue - are similar between originators and biosimilars. Some studies show a slight increase in mild skin reactions with certain adalimumab biosimilars, but this doesn’t mean the drug is less safe. Often, patients report new side effects due to the nocebo effect - anxiety about the switch triggers perceived symptoms. Real immune reactions are rare, occurring in fewer than 2% of patients per year.
Can I switch back to the original drug if I don’t feel well?
Yes. If you feel worse after switching, talk to your doctor. They can request a medical exception from your insurance to return to the originator biologic. Many patients do this successfully. But it’s important to rule out other causes first - like stress, infection, or changes in diet - before assuming the drug is the problem. Your doctor may check your drug levels or antibodies to help decide.
What’s the difference between biosimilar and generic?
Generics are chemically identical copies of small-molecule drugs, like ibuprofen or metformin. Biosimilars are copies of large, complex biologic drugs made from living cells. Because they’re made by living systems, biosimilars can have tiny structural differences - but they must perform the same way in the body. The FDA requires much more testing for biosimilars than for generics, including clinical trials to prove they work the same.
Is it safe to switch between different biosimilars?
Current evidence suggests it’s generally safe. Studies like the one by Lauret et al. in 2022 show patients can switch from one biosimilar to another - such as from CT-P13 to SB2 - without increased risk of immune reactions or loss of effectiveness. Drug levels in the blood stay stable, and adverse events don’t rise. However, multiple switches haven’t been studied over very long periods, so some doctors prefer to limit switches to one or two, especially in patients with complex disease histories.
Why do some patients stop taking biosimilars if they’re safe?
Many stop because they believe the biosimilar won’t work as well - even when it does. This is called the nocebo effect. Studies show up to one-third of patients report new symptoms after a switch, despite normal test results. Other reasons include injection discomfort, insurance forcing the switch without discussion, or confusion about the change. Real drug failure is rare. Most discontinuations are due to perception, not biology.
What Comes Next?
The future of biosimilars is growing fast. More originator biologics are losing patent protection through 2025 - including key drugs for multiple sclerosis, asthma, and diabetes. That means more biosimilars will enter the market, driving prices down further.
But the biggest challenge isn’t science. It’s trust. Patients need to know their doctors aren’t cutting corners. Pharmacists need clear guidance. Insurers need to stop forcing switches without input.
If you’re switching, don’t go it alone. Ask questions. Track your body. Speak up if something feels off. You’re not just a data point. You’re the most important part of the treatment.






10 Comments
I switched from Humira to an adalimumab biosimilar last year and honestly? I didn’t notice a difference. My DAS28 stayed the same, no new flares, no weird side effects. But I did feel this weird anxiety before the switch - like I was betraying my old meds. It’s wild how much your brain can make you feel things that aren’t there.
Now I keep a little journal. Just pain levels and fatigue. Helps me separate real changes from fear.
Also, my rheum doc gave me 20 minutes of counseling before the switch. That tiny chat made all the difference. Seriously, ask for it.
YASSS to informed switching!! 🙌
So proud of how far we’ve come with biosimilars - they’re not ‘cheap knockoffs,’ they’re science miracles 💫
My cousin was terrified to switch but now she’s thriving and saving $$$ - and her insurance didn’t even make her fight for it! 🌟
Education + trust = WINNING combo. Keep pushing the narrative, docs and patients alike!!
I get why people are nervous. I was too. But after reading all the studies and talking to my GI, I realized most of my fear came from not knowing. Once I understood that biosimilars aren’t ‘different’ - just cheaper versions of the same thing - it felt less scary.
My joints didn’t flare. My skin didn’t breakout. My bank account? Definitely happier.
Maybe the real issue isn’t the drug… it’s how we talk about it.
Let’s be real - this whole biosimilar push is Big Pharma’s latest scam to offload old inventory. You think the FDA really tested these things properly? They’re cutting corners. And now they’re letting pharmacists swap them out without even asking you?
I’ve seen people go from stable to hospitalized after a switch. No one talks about that. The system doesn’t want you to know the truth.
They’re playing with lives for profit. And you’re just… accepting it?
To everyone scared of switching: you’re not alone. I’ve been there.
But here’s what I learned - the data doesn’t lie. Over 120 studies. Real people. Real outcomes. Biosimilars work.
And yes, some people feel worse - but it’s rarely because the drug failed. It’s because they were told to expect failure.
If you’re switching, ask for a counseling session. Write down how you feel. Talk to others who’ve done it. You’re not a guinea pig. You’re part of a movement making life-saving meds affordable.
And if something feels off? Speak up. You have rights. You’re not powerless.
We’ve got this.
USA is falling behind because we let corporations run everything. In Germany, they’ve been switching for years. No drama. No fear. Just better prices and same results.
Here? We’re stuck in patent lawsuits and rebate traps. And now they want us to believe biosimilars are dangerous? LOL.
It’s not the drugs. It’s the greed. And it’s not just biologics - it’s insulin, it’s vaccines, it’s everything.
Stop letting them gaslight you. The science is solid. The system is broken.
Bro i switched to biosimilar last year after my doc said its same thing just cheaper
no change in my crohns at all
my wife said i was gonna die but i lived
now i tell everyone its fine
why u scared if ur body dont feel different
also saved like 1200 a month
usa prices are wild
in india we just get it cheap no drama
why we make it hard here
peace
They’re lying to you. Biosimilars aren’t safe. The FDA is in bed with Big Pharma. They’re testing these drugs on people like us to see how many get sick before they pull them.
My cousin’s mom switched and got cancer 6 months later. Coincidence? I don’t think so.
They don’t want you to know that the original drug has 30 years of history. Biosimilars? 5 years. That’s not science. That’s a gamble.
And now they’re letting pharmacists swap them without your doctor’s say-so? That’s not healthcare. That’s corporate warfare.
Don’t let them turn you into a test subject.
Just to clarify something - biosimilars aren’t generics because biologics are made from living cells, not synthesized in a lab. That’s why they’re more complex and require more testing.
But the data is solid. Multiple studies show no clinically meaningful difference in efficacy or safety. The EMA and FDA both agree.
Side effects? Mostly injection site reactions - similar to originators. Anti-drug antibodies? Extremely rare - under 2% per year.
The real issue is perception. If you’re told the drug is ‘inferior,’ your brain will make you feel it - even if it’s not true.
Knowledge reduces fear. Talk to your doctor. Read the studies. Don’t let misinformation scare you.
yo i switched from humira to a biosimilar last year and honestly i thought i was gonna die
but nothing happened
my skin stayed clear
my knees stopped creaking
and i saved like 1500 a month
my doc said its the same thing just cheaper
and i was like wait… so they’ve been charging me 2k for the same drug?
why is america like this
also my pharmacist tried to swap me again without telling me
and i was like NOPE
you gotta ask me first
we need better info and less corporate BS
thanks for the post btw