Tag: FDA guidance 2025

Biosimilar Approval: How the FDA Reviews Biologic Alternatives in 2026
The FDA's 2025 guidance streamlines biosimilar approval by replacing costly clinical trials with advanced analytical data, accelerating access to lower-cost biologic alternatives for cancer, arthritis, and diabetes. Learn how the process works and what's still holding it back.
view morerecent posts

Generic drugs cost far less than brand-name drugs not because ingredients are cheaper, but because labor costs are structured differently. Learn how scale, regulation, and global production shape the real cost of your prescriptions.
Published ON: 9 Feb
Brand companies launch authorized generics to protect revenue when patents expire. These are identical to the original drug but sold at lower prices, undercutting competitors and keeping patients loyal. It’s a smart business move - and it saves consumers money.
Published ON: 23 Feb
Learn how post-marketing studies track drug safety after approval, using FAERS, Sentinel, and real-world data to catch hidden risks. Understand the systems, challenges, and future of pharmacovigilance.
Published ON: 11 Feb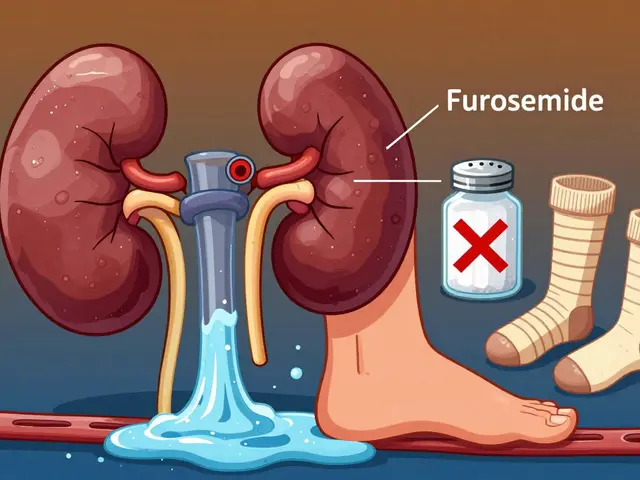
Edema in CKD is caused by fluid buildup from failing kidneys. Managing it requires a three-part strategy: the right diuretics, strict salt restriction, and compression therapy. Learn how each works-and how to use them safely.
Published ON: 7 Feb
Most workplace wellness programs fail because employees don’t understand how they benefit. Learn how to educate staff with clear, personalized messaging that boosts participation, cuts costs, and builds trust.
Published ON: 1 Feb