Tag: ANDA application

Generic Drug User Fee Amendments: How GDUFA Laws Speed Up FDA Review
GDUFA laws let the FDA collect fees from generic drug makers to speed up approval of affordable medications. Since 2012, review times have dropped from years to months, helping millions access low-cost drugs faster.
view morerecent posts

Most workplace wellness programs fail because employees don’t understand how they benefit. Learn how to educate staff with clear, personalized messaging that boosts participation, cuts costs, and builds trust.
Published ON: 1 Feb
Discover how the FDA maintains strict quality standards for generic drugs through cGMP regulations, unannounced inspections, and rigorous testing. Learn about the five key control areas, global comparisons, and real-world impact on drug safety and affordability.
Published ON: 4 Feb
Learn how post-marketing studies track drug safety after approval, using FAERS, Sentinel, and real-world data to catch hidden risks. Understand the systems, challenges, and future of pharmacovigilance.
Published ON: 11 Feb
Learn how to verify online pharmacy licenses to avoid counterfeit drugs. Step-by-step guide to using state and NABP verification systems for safe medication purchases.
Published ON: 15 Feb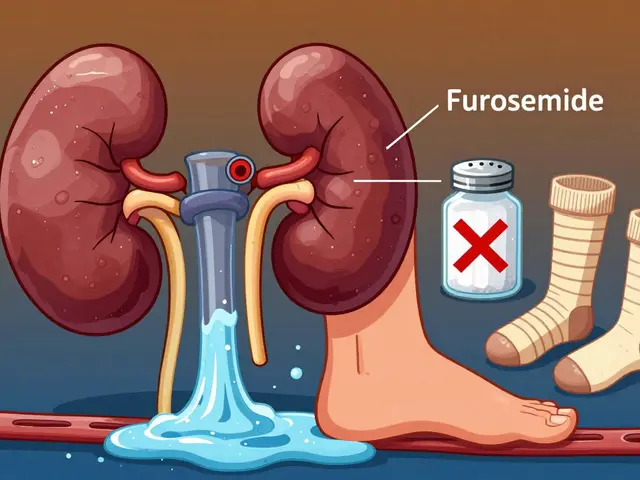
Edema in CKD is caused by fluid buildup from failing kidneys. Managing it requires a three-part strategy: the right diuretics, strict salt restriction, and compression therapy. Learn how each works-and how to use them safely.
Published ON: 7 Feb