Corrective Actions in Pharmacy: How Regulators Fix Drug Safety Issues
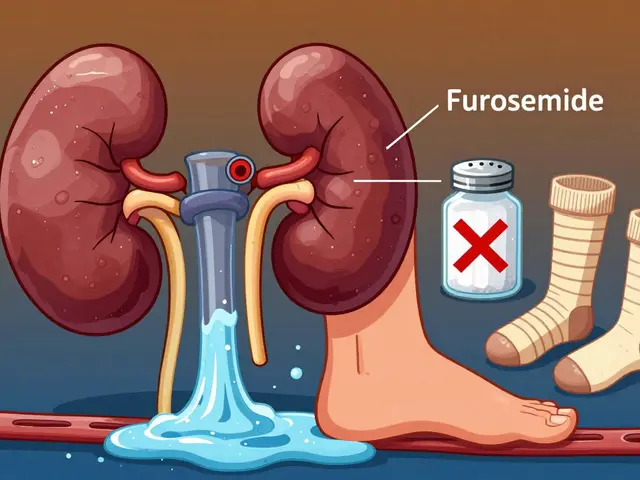
When something goes wrong with a drug—whether it’s a contaminated batch, false labeling, or unsafe manufacturing—corrective actions, steps taken by regulators to fix violations and protect public health. These aren’t just paperwork. They’re the system’s way of stepping in before someone gets hurt. You might not see them, but they’re happening every day. The FDA, the U.S. agency that oversees drug safety and approves medications issues warning letters to companies that break the rules. These aren’t threats—they’re official notices that demand change. If a company ignores them, the FDA can pull products off shelves, block imports, or even push for criminal charges.
Non-compliant manufacturers, pharmaceutical companies that fail to meet quality or safety standards are the main targets. Maybe they didn’t clean equipment properly, mixed up ingredients, or hid side effects. The FDA doesn’t wait for harm to happen. They use inspections, adverse event reports from doctors and patients, and data from systems like MedWatch, the FDA’s system for tracking bad reactions to drugs to find problems early. Once they spot a pattern—say, a generic drug causing more liver issues than it should—they demand corrective actions. That could mean recalling pills, fixing the production line, or retraining staff.
It’s not just about big pharma. Even small online pharmacies that sell meds without proper licensing can trigger corrective actions. If a site sells expired drugs or fake versions of popular meds, regulators step in. The goal isn’t punishment—it’s prevention. Every corrective action is meant to stop the next person from getting sick because of a bad pill. These actions keep your medicine safe, even when you’re buying online. And they’re why you should only trust pharmacies that follow clear, verifiable standards.
What you’ll find below are real stories of what happens when things go wrong—and how the system responds. From FDA warning letters to how generic drugs are monitored after they hit the market, these posts show the hidden work behind every safe prescription you take.
How Manufacturers Fix Quality Problems: A Practical Guide to Corrective Actions
Manufacturers fix quality problems by finding root causes, not just symptoms. Learn how CAPA systems, root cause analysis, and verification steps reduce defects, ensure compliance, and prevent recurrence in regulated industries.
view more