Tag: biologic alternatives

Biosimilar Approval: How the FDA Reviews Biologic Alternatives in 2026
The FDA's 2025 guidance streamlines biosimilar approval by replacing costly clinical trials with advanced analytical data, accelerating access to lower-cost biologic alternatives for cancer, arthritis, and diabetes. Learn how the process works and what's still holding it back.
view morerecent posts

Most workplace wellness programs fail because employees don’t understand how they benefit. Learn how to educate staff with clear, personalized messaging that boosts participation, cuts costs, and builds trust.
Published ON: 1 Feb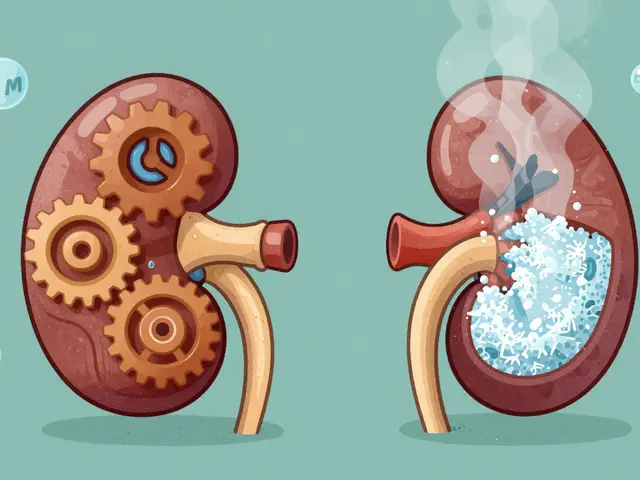
Hyponatremia and hypernatremia are common, dangerous complications in kidney disease. Learn how sodium imbalances happen, why standard treatments fail, and what actually works to protect your health.
Published ON: 8 Feb
Learn how to use pharmacy apps to catch dangerous drug interactions before they harm you. From free tools like Drugs.com to professional apps like Epocrates and Lexicomp, find out which ones work best and how to use them safely.
Published ON: 25 Feb
Brand companies launch authorized generics to protect revenue when patents expire. These are identical to the original drug but sold at lower prices, undercutting competitors and keeping patients loyal. It’s a smart business move - and it saves consumers money.
Published ON: 23 Feb
Generic drugs cost far less than brand-name drugs not because ingredients are cheaper, but because labor costs are structured differently. Learn how scale, regulation, and global production shape the real cost of your prescriptions.
Published ON: 9 Feb