
Every year, over 90% of prescriptions in the U.S. are filled with generic drugs-cheaper, equally effective, and approved by the FDA. But sometimes, a doctor says: no. Not this time. I need the brand name. That’s not a suggestion. It’s a legal override. And if the pharmacist ignores it, they could be breaking the law.
Why Do Doctors Override Generic Substitution?
It’s not about profit. It’s not about preference. It’s about safety. Some drugs have what’s called a narrow therapeutic index. That means the difference between a dose that works and a dose that kills is tiny. A small change in how the drug is absorbed-maybe because of a different filler or coating in the generic version-can throw a patient into crisis. Take levothyroxine. It’s the most common thyroid medication. The FDA says generics are bioequivalent. But patients on stable doses can suffer thyroid storm-life-threatening hyperthyroidism-if they switch to a different generic batch. A 2022 case study in Michigan documented a patient hospitalized after a pharmacy substituted a different generic version, even though the doctor had written "Dispense as Written." The patient’s heart rate spiked to 160 beats per minute. That’s not a rare event. The Institute for Safe Medication Practices tracked 27 serious adverse events between 2018 and 2022 linked to improper substitutions of warfarin, phenytoin, or levothyroxine. Other cases include epilepsy patients on phenytoin. A 0.5 mg/L shift in blood levels can trigger seizures. Psychiatric drugs like lithium and certain antidepressants also fall into this high-risk category. When a patient has tried a generic and had a relapse, a doctor’s override isn’t stubbornness-it’s clinical necessity.How the Override Actually Works
It all comes down to a code: DAW-1. That’s short for Dispense as Written. When a doctor writes this on a prescription, they’re telling the pharmacy: do not substitute. But here’s the catch-DAW-1 doesn’t mean the same thing everywhere. In Illinois, the prescriber must check a box labeled "May Not Substitute." In Kentucky, they must write "Brand Medically Necessary" by hand. In Massachusetts, "No Substitution" works. In Oregon, a phone call is enough. Michigan requires the words "DAW" or "Dispense as Written" written clearly. And in Texas, the prescription must follow a strict two-line format under state code 295.201. If the doctor doesn’t follow the exact state rule, the pharmacy is legally allowed to substitute anyway. And that’s where things go wrong. A 2022 survey of 1,247 physicians on Sermo found that 41% had prescriptions rejected because their documentation didn’t match their state’s requirements. One doctor in Florida wrote "Do Not Substitute"-but Florida requires "Brand Medically Necessary." The pharmacy substituted the generic. The patient’s blood pressure spiked. They ended up in the ER.The Role of the Orange Book
The FDA doesn’t force pharmacists to substitute. But they do publish the Approved Drug Products with Therapeutic Equivalence Evaluations-better known as the Orange Book. This is the official list that tells pharmacists which generics are considered therapeutically equivalent to brand-name drugs. Each drug gets an "A" or "B" rating. "A" means it’s safe to swap. "B" means it’s not. Here’s the twist: 42 states require pharmacists to check the Orange Book before substituting. But if the doctor writes DAW-1, the pharmacist doesn’t even need to look. The override trumps the book. That’s why it’s so critical for doctors to understand what the Orange Book says. If a drug has a "B" rating, even if the doctor doesn’t override, the pharmacist shouldn’t substitute. But most doctors don’t know this. A 2010 study found only 58.3% of physicians understood their state’s override rules.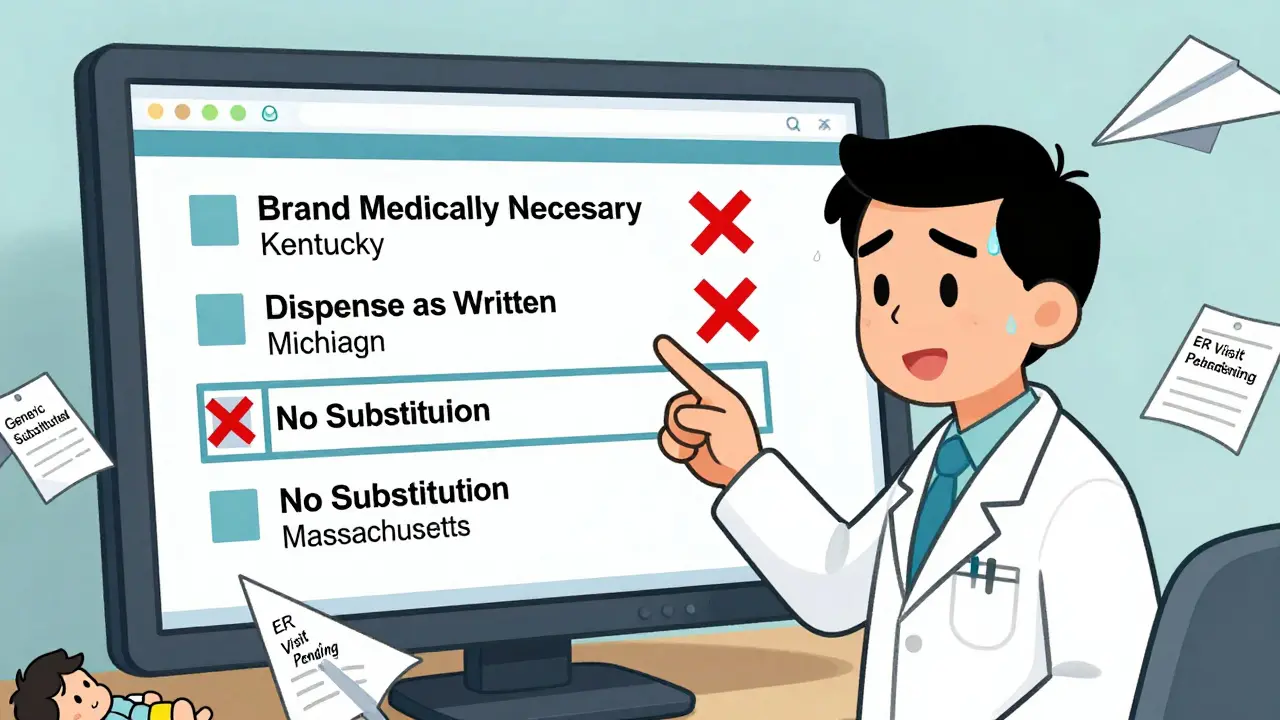
The Cost of Overrides
Generics saved the U.S. healthcare system $2.2 trillion between 2010 and 2019. That’s huge. But every time a doctor writes DAW-1, the cost jumps. On average, a DAW-1 prescription costs 32.7% more than a substituted generic. In 2021, Express Scripts found that 18.4% of brand-drug spending was avoidable-because of inappropriate overrides. That’s not just about insurance companies. It’s about patients. High copays mean people skip doses. One patient in Alabama told her doctor she stopped her brand-name blood pressure pill because the copay was $85. She switched to the generic, didn’t tell her doctor, and ended up with a stroke. The override was meant to protect her-but without proper communication, it backfired. The American Pharmacists Association estimates that 5-7% of prescriptions truly need an override. But studies show that up to 30% of DAW-1 requests are unnecessary. Why? Because many doctors don’t realize how similar generics really are. A 2019 JAMA Internal Medicine analysis found that physicians often overestimate the clinical impact of minor formulation differences. They think the generic is "different," when the data says it’s not.Electronic Prescribing and the Real-World Mess
Most prescriptions are sent electronically now. But EHR systems are a mess. Many default to generic substitution unless the doctor actively changes it. And the templates? They’re outdated. One doctor in California spent 10 minutes trying to figure out why her override request kept getting rejected. Her EHR had a drop-down menu with "Do Not Substitute"-but California law requires "Brand Medically Necessary." The system didn’t recognize her wording. The pharmacy substituted anyway. A 2021 study found that EHRs without customized override templates added 1.7 minutes per prescription. With proper setup, that drops to under a minute. But most clinics don’t have the resources to customize their systems. And pharmacies? They’re overwhelmed. A nurse on AllNurses reported that 68% of override rejections come from sloppy documentation-handwritten notes that don’t scan well, missing signatures, unclear handwriting.
What Doctors Need to Do Right Now
If you’re a physician, here’s what you need to do:- Know your state’s override rules. Go to the National Association of Boards of Pharmacy website. They have an interactive map updated quarterly.
- Use the exact wording your state requires. Don’t improvise. "Dispense as Written" isn’t enough in some places. "Brand Medically Necessary" might be mandatory.
- Check the Orange Book for drugs with "B" ratings. If it’s a "B," don’t even consider substitution-override or not.
- Ask your pharmacy what they need. Some want a signature. Others need a note on the electronic form. Don’t assume.
- Train your staff. Nurses and medical assistants often enter the prescription. They need to know the rules too.
The Future: One Rule for All?
Right now, we have 50 different rulebooks. A doctor in New York who sees patients in Pennsylvania has to learn two sets of rules. That’s why Congress introduced the Standardized Prescriber Override Protocol Act in 2023. It’s still pending, but the goal is simple: one national standard for DAW-1. The FDA is also updating the Orange Book. Version 4.0, released in January 2023, now includes biosimilars-meaning future overrides might apply to biologic drugs like Humira or Enbrel. That’s a big deal. These drugs cost over $100,000 a year. If a patient switches to a biosimilar and has a reaction, the consequences are severe. Meanwhile, the NCPDP is building override rules directly into the next version of e-prescribing software. By late 2024, systems should auto-populate the correct state language based on the prescriber’s location.Bottom Line: Overrides Are Necessary-But Only When Used Right
Prescriber override isn’t a loophole. It’s a safety valve. It exists because medicine isn’t one-size-fits-all. But when it’s used carelessly-because of ignorance, laziness, or habit-it becomes a financial drain and a patient risk. The goal isn’t to eliminate overrides. It’s to make them precise. When done right, they prevent hospitalizations. When done wrong, they cause them. Doctors need to treat DAW-1 like a surgical instrument-not a checkbox. Use it only when the science says you must. And always, always follow the rules.Can a pharmacist refuse to fill a DAW-1 prescription?
No. If a prescriber properly documents a DAW-1 override according to state law, the pharmacist is legally required to dispense the brand-name drug. Refusing to do so could result in disciplinary action by the state board of pharmacy. However, if the override is improperly documented (e.g., missing required wording or signature), the pharmacist may legally substitute the generic.
Do I need to override for every generic drug?
No. Most generic drugs are safe and effective substitutes. Overrides should be reserved for medications with a narrow therapeutic index-like warfarin, levothyroxine, phenytoin, lithium, or certain anti-seizure drugs. For common drugs like atorvastatin or metformin, generic substitution is not only acceptable-it’s standard practice.
What happens if I write "no substitution" but my state requires "Brand Medically Necessary"?
The pharmacy may still substitute the generic. State laws are very specific about wording. If your prescription doesn’t match the exact phrase required by your state, the pharmacist is not obligated to honor your request. Always check your state’s pharmacy board guidelines before writing an override.
Can patients request brand-name drugs even if the doctor didn’t override?
Yes. That’s called DAW-2. If a patient insists on the brand name-even when the doctor didn’t require it-the pharmacist can dispense it, but the patient will usually pay the full brand price. Insurance won’t cover the difference unless the patient has a prior authorization or meets specific criteria.
Are there any drugs that can never be substituted, even without a DAW-1?
Yes. The FDA’s Orange Book assigns "B" ratings to drugs that are not considered therapeutically equivalent. These include certain extended-release formulations, complex generics, or drugs with unclear bioequivalence data. Pharmacists are not allowed to substitute these, even if no override is written. Always check the Orange Book for drugs you’re unsure about.






9 Comments
Been a pharmacist for 12 years. Saw a guy go into atrial fibrillation because his levothyroxine was switched without the doctor's DAW-1. We got called out for it. Turned out the doctor had written it but used the wrong phrase. No one wins when paperwork is sloppy.
My mom’s on lithium. One generic switch and she was in the ER for three days. I don’t care what the Orange Book says-when your life’s on the line, you don’t gamble with fillers and coatings. DAW-1 isn’t a luxury. It’s survival.
As a med student who’s shadowed ER docs, I’ve seen the fallout from bad substitutions. Phenytoin levels shifting by 0.5 mg/L? That’s not a typo-that’s someone seizing in a parking lot. Doctors aren’t being stubborn. They’re the last line between a patient and disaster. We need better EHR templates, not fewer overrides.
Big Pharma is laughing all the way to the bank. They pay doctors to push brand names under the guise of safety. The FDA’s Orange Book is a joke. The real reason generics get rejected? Insurance companies won’t cover the brand unless you jump through 17 hoops. Meanwhile, patients die because their prescriptions get lost in bureaucracy. This whole system is rigged.
It is of paramount importance to recognize that the legal and pharmacological frameworks governing therapeutic substitution are not merely procedural formalities but are instead foundational to the integrity of public health policy. The variance in state-specific requirements for DAW-1 documentation creates a regulatory patchwork that is not only inefficient but inherently hazardous, as evidenced by the documented cases of iatrogenic harm resulting from noncompliant prescriptions. Furthermore, the failure of electronic health record systems to dynamically align with jurisdictional mandates constitutes a systemic failure of technological governance in clinical practice.
I work in a clinic and we just got our EHR updated. Now it auto-fills the right wording based on our state. Took us 6 months to get it right but now we don’t get rejected anymore. Small change, huge difference. Maybe more clinics should ask their vendors for this
So let me get this straight-if a doctor writes 'no substitution' but the state says 'Brand Medically Necessary' then the pharmacist can just give the generic and no one’s at fault? That’s insane. It’s like saying 'you have to say 'red light' not 'stop' or the cop can still shoot you.' This isn’t medicine, it’s a legal trap. Doctors aren’t lawyers. Why should they have to memorize 50 different rulebooks?
As someone who’s worked in rural clinics across three states, I’ve seen this play out over and over. A patient gets their thyroid med switched because the doctor didn’t know the state’s wording. They come back pale, shaky, heart racing. We fix it, but the damage is done. The real issue isn’t the override-it’s that we train doctors to prescribe but not to navigate pharmacy law. We need mandatory continuing ed on this. Not optional. Required.
Why do Americans think their system is better? In South Africa, we don’t have this mess. Generics are generics. If it works, you take it. If it doesn’t, you see the doctor. No paperwork. No lawsuits. No drama. Maybe the U.S. needs less bureaucracy and more trust in science.