18 Dec |
11:35 AM
When you’re fighting cancer or an autoimmune disease, the last thing you expect is for an old virus to come back and attack your liver. But that’s exactly what happens in HBV reactivation - a silent, deadly risk hidden inside routine treatments like chemotherapy and biologic drugs. It’s not rare. It’s not theoretical. It’s happened to real people, often because no one checked for hepatitis B before starting treatment.
What Exactly Is HBV Reactivation?
Hepatitis B virus (HBV) doesn’t always go away after infection. For many, it hides in the liver, quiet and inactive. This is called chronic infection if you’re HBsAg-positive, or resolved infection if you’re HBsAg-negative but anti-HBc-positive. Your immune system keeps it in check - until something weakens it. That’s when reactivation kicks in. Immunosuppressive drugs - the very ones meant to calm your immune system to treat cancer or rheumatoid arthritis - accidentally remove the brakes on HBV. The virus starts multiplying. Liver cells get attacked. ALT and AST levels spike. You develop jaundice, fatigue, nausea. In severe cases, it leads to liver failure or death. This isn’t a new problem. Doctors first noticed it in the 1970s with chemotherapy. But it exploded in the 2000s with biologics like rituximab. A 2016 study in Blood found that 38-73% of HBsAg-positive lymphoma patients on rituximab had reactivation. Without treatment, up to 10% of those cases were fatal.Who’s at Risk - And How Much?
Not everyone faces the same danger. Your risk depends on two things: your HBV status and the drug you’re taking.- HBsAg-positive (active infection): Highest risk. Even with no symptoms, your liver is a ticking time bomb under immunosuppression.
- HBsAg-negative, anti-HBc-positive (past infection): Lower, but still real. Up to 18% can reactivate, especially with strong drugs like rituximab or high-dose chemo.
- Anti-HBs-positive (immune from vaccine or cleared infection): Very low risk - under 1%.
- High-risk (20-81% reactivation): Anti-CD20 drugs (rituximab, ofatumumab), anthracycline chemo, stem cell transplants.
- Intermediate-risk (1-10%): TNF-alpha inhibitors (infliximab, adalimumab), ibrutinib, radiation therapy for liver cancer.
- Low-risk (<1%): Most non-TNF biologics, non-cytotoxic targeted therapies.
Why Screening Is Non-Negotiable
The good news? This is 95% preventable. The bad news? Too many people still aren’t screened. Every major guideline - from the American Association for the Study of Liver Diseases (AASLD), the European Association for the Study of the Liver (EASL), and the Infectious Diseases Society of America (IDSA) - says the same thing: Test everyone before starting immunosuppressive therapy. You need two simple blood tests:- HBsAg - tells you if the virus is currently active.
- Anti-HBc - tells you if you’ve ever been infected.

How Prophylaxis Works - And When to Start
The go-to drugs are tenofovir and entecavir. Both are potent, safe, and taken as one pill a day. They don’t cure HBV - but they stop it from exploding during treatment. Timing matters:- Start antivirals at least one week before immunosuppression begins.
- Continue for 6 to 12 months after treatment ends - longer for high-risk drugs like rituximab or stem cell transplants.
Real Cases - What Happens When You Skip Screening
A 52-year-old man with lymphoma got rituximab. No HBV test. Two weeks in, he got jaundice. By week four, his liver was failing. He died. His family didn’t know he’d had hepatitis B as a child. Another case: a woman with rheumatoid arthritis started adalimumab. She was HBsAg-negative, anti-HBc-positive. No prophylaxis. She developed severe hepatitis. Her ALT jumped to 1,200. She needed a transplant. These aren’t outliers. They’re preventable tragedies. The Hepatology Communications case report from 2019 called it a “failure of system-level care.” On the flip side, UCSF Medical Center cut reactivation rates from 12.3% to 1.7% in just five years - by adding automatic alerts in their electronic health records. Every patient getting chemo or biologics got flagged for HBV testing. No exceptions.Why This Isn’t Just About Liver Health
HBV reactivation isn’t just a liver problem. It’s a cancer treatment killer. It derails chemotherapy cycles. It forces hospitalizations. It adds tens of thousands in extra costs. The global HBV screening market is projected to hit $612 million by 2027. Why? Because hospitals are finally realizing the cost of not screening is higher than the cost of testing. In 2019, HBV reactivation made up 12% of infectious complication claims in oncology malpractice cases. The FDA now requires HBV warnings on all immunosuppressive biologic labels. That’s not a formality - it’s a legal requirement because the risk is proven, predictable, and preventable.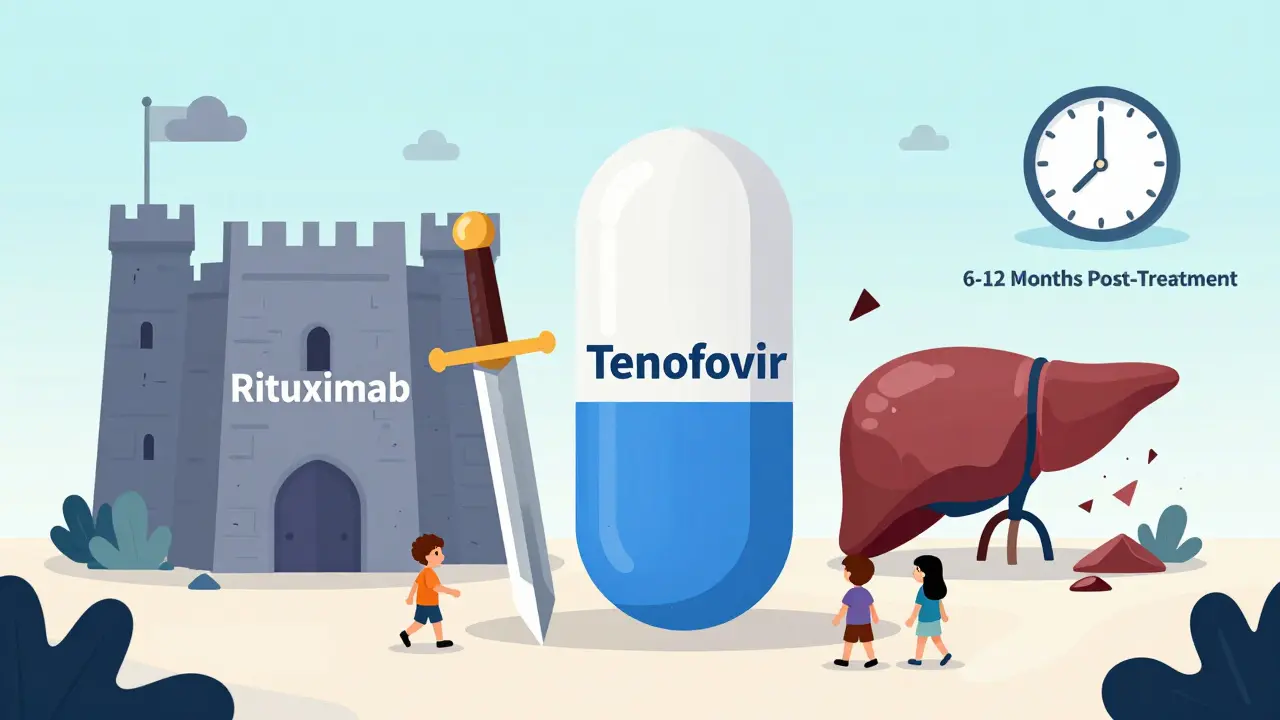
What You Should Do - Step by Step
If you’re about to start chemotherapy, biologics, or any strong immunosuppressant:- Ask your doctor: “Have I been tested for hepatitis B?” If they say no, insist.
- Get HBsAg and anti-HBc tested - at least two weeks before treatment starts.
- If HBsAg is positive: You’ll start tenofovir or entecavir immediately.
- If HBsAg-negative but anti-HBc-positive: Ask if your treatment is high-risk. If yes, you need prophylaxis too.
- Don’t stop antivirals early. Even if you feel fine, the virus can flare after treatment ends.



15 Comments
This hit me hard. My aunt went through chemo last year and never got tested. She ended up in the hospital with liver failure. No one even asked about hepatitis B. I wish I’d known this sooner. Please, if you’re about to start treatment - ask. Just ask.
It’s not complicated. Two blood tests. One pill. Could save your life.
Thanks for writing this.
I work in a clinic and we started doing mandatory HBV screens before biologics last year. Guess what? We caught three silent carriers who would’ve crashed hard on rituximab. One guy thought he was ‘immune’ because he got the vaccine as a kid. Nope. Anti-HBc positive. He’s on tenofovir now. No drama.
System changes save lives. Simple as that.
EVERYONE KNOWS THIS IS A COVER-UP. The pharma giants don’t want you to know how many people they’re killing with these drugs - because if you knew, you’d sue them into oblivion. They bury the data. They bury the warnings. They bury the尸骸.
And now they’re pushing ‘rapid tests’ like it’s a miracle? HA! It’s a distraction. They’re just trying to look responsible while they keep raking in billions. The FDA? Controlled. The guidelines? Paid for. Your liver? Disposable.
Don’t trust the system. Test yourself. Then run.
And bring a flashlight. The dark is coming.
What’s the metaphysical cost of medical paternalism? We outsource our agency to institutions that treat our bodies like statistical noise. HBV reactivation isn’t just a clinical event - it’s the embodiment of systemic indifference.
When a patient must beg for a basic blood test to avoid death, we’ve abandoned medicine’s moral core. We’ve turned healing into a transaction, and life into a risk-adjusted probability.
The pill works. The test works. But the will to act? That’s the real epidemic.
And until we confront that - we’re all complicit.
Okay, let’s get real. You’re telling me 48% of untreated HBsAg+ patients reactivate? That’s not a statistic - that’s a massacre. And yet, in India, most oncologists still don’t screen unless the patient is visibly jaundiced. We have a 10-year-old WHO guideline in a drawer somewhere, covered in dust and bureaucracy.
And don’t even get me started on how ‘anti-HBc positive’ patients get ignored because ‘it’s low risk’ - until they’re on a transplant list.
My cousin died because her rheumatologist said ‘you’re fine’ after a 30-second chat. No test. No follow-up. Just ‘trust me.’
So yeah. Ask. Push. Don’t be polite. Be loud.
Let me break this down for the slow folks: If you're on rituximab and haven't been tested for HBV, you're playing Russian roulette with your liver. Not 'maybe' - not 'possibly' - you're literally gambling with your life. And if your doctor says 'we don't do that here' - fire them. Find someone who actually reads the guidelines. Or Google 'AASLD HBV prophylaxis' and print it out. Hand it to them. Then sit there until they sign off.
This isn't advice. It's a survival protocol.
OMG I just had a panic attack reading this 😭 I’m starting adalimumab next week and I’ve NEVER been tested. I’m calling my doctor right now. Thank you for posting this. I literally would’ve died without knowing. 🙏❤️
Correction: The 2022 NEJM study showed that six months of post-treatment prophylaxis was non-inferior to 12 months in patients receiving anti-CD20 therapy, with hazard ratio 0.89 (95% CI 0.61–1.30). The reduction in duration applies only to patients without prior cirrhosis or ongoing immunosuppression. For stem cell transplant recipients, 12 months remains standard.
Also, entecavir is preferred over tenofovir in patients with renal impairment. Tenofovir disoproxil fumarate (TDF) carries higher nephrotoxicity risk than tenofovir alafenamide (TAF).
These nuances matter. Don’t generalize.
Screening is necessary. Prophylaxis works. People die without it. End of story.
Stop overthinking it. Test. Treat. Move on.
And if your doctor doesn't do it? Go somewhere else.
Simple
I love how this post is both terrifying and empowering. Like… yeah, this could kill you. But also, you have all the power to stop it. Just two tests. One pill. No magic. No mystery.
It’s the kind of thing that makes you want to scream at the world - and then quietly text your cousin who’s about to start chemo.
Thank you for writing this. I’m sharing it everywhere.
As a nurse who’s seen three patients die from this exact thing - I can tell you the system fails daily. We have protocols. We have guidelines. We have the drugs. But too many providers still think ‘HBV screening’ means ‘ask if they’re Asian.’
It’s not about ethnicity. It’s about exposure. It’s about history. It’s about a blood test.
I’ve started handing out printed checklists to every patient starting biologics. One page. Two tests. One question: ‘Have you been screened?’
It’s not glamorous. But it saves lives.
And if you’re reading this and you’re scheduled for treatment - don’t wait. Ask now. Not tomorrow. Now.
Man, this is one of those rare posts that actually makes you feel like you’ve learned something that could save your life. I’ve been on adalimumab for years and never thought twice about HBV. Now I’m getting tested tomorrow. No excuses. No ‘maybe later.’
Thanks for being the voice that says what nobody else will. This is the kind of content that should be mandatory reading for every patient on immunosuppressants.
It is an incontrovertible fact that the prevalence of occult hepatitis B infection among populations with prior exposure exceeds 10% in endemic regions, and the immunosuppressive milieu induced by biologics precipitates viral replication with alarming predictability. The failure to implement universal screening protocols constitutes a gross dereliction of medical duty, particularly in light of the cost-effectiveness of antiviral prophylaxis, which reduces reactivation rates by over 90% and averts hospitalization expenditures exceeding $25,000 per episode.
It is therefore not merely a clinical recommendation - it is an ethical imperative.
Bro this is wild 😳 I thought HBV was just a thing from the 90s. I got the vaccine as a kid so I thought I was good to go. Turns out I’m anti-HBc positive and I’m starting infliximab next month. I’m calling my doc right now. Thanks for the wake-up call 🙌💊
I’m a nurse in Mumbai. We don’t have the resources to screen everyone. But we started a simple rule: if you’re on anything stronger than methotrexate - we test. No exceptions. We’ve cut reactivation cases by 80% in two years.
It’s not about money. It’s about will.
If you’re reading this and you’re in a position to push for change - do it. Even one test. One pill. One life saved.
It’s worth it.