Tag: FAERS

How to Track Post-Marketing Studies for Drug Safety
Learn how post-marketing studies track drug safety after approval, using FAERS, Sentinel, and real-world data to catch hidden risks. Understand the systems, challenges, and future of pharmacovigilance.
view morerecent posts
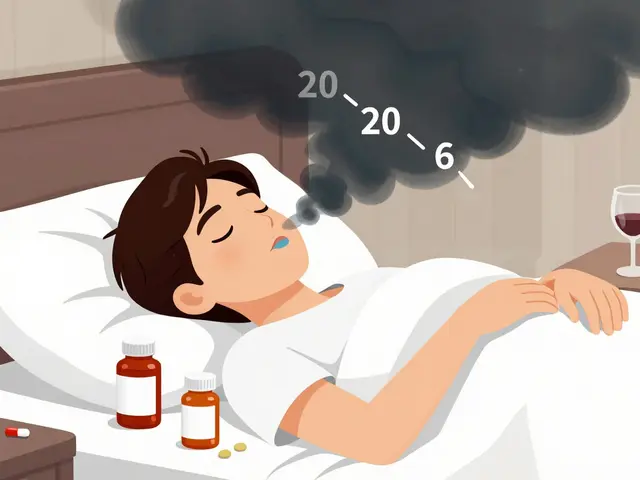
Combining Multiple Sedating Medications: Risks and Warning Signs
Combining sedating medications like opioids and benzodiazepines can lead to deadly respiratory depression. Learn the warning signs, dangerous combinations, and how to reduce your risk safely.
Published ON: 1 Mar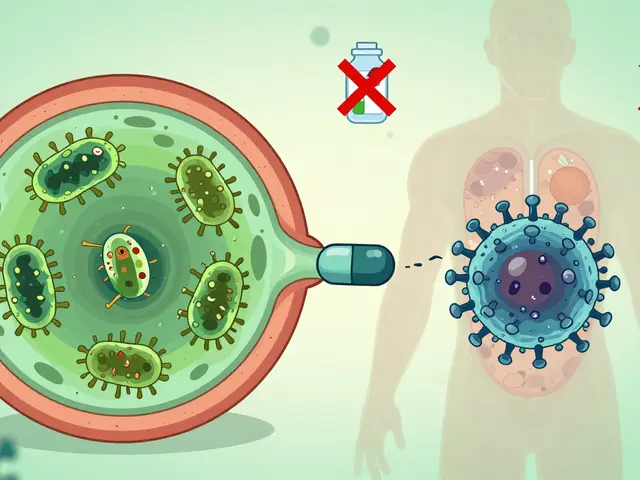
Bacterial vs. Viral Infections: Key Differences and Treatments
Bacterial and viral infections look similar but require completely different treatments. Antibiotics work on bacteria, not viruses-and misuse fuels dangerous drug resistance. Learn how to tell them apart and what to do when you're sick.
Published ON: 2 Mar